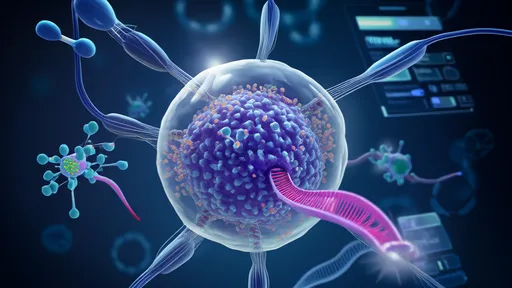
The field of mitochondrial transplantation has entered a bold new phase with the advent of what researchers are calling "Mitochondrial Transplantation 2.0." This cutting-edge approach moves beyond early experimental therapies by leveraging stem cells as universal donors for these vital energy-producing organelles. At its core, the technology challenges biological boundaries through cross-species compatibility – a concept that could rewrite textbooks on cellular compatibility.
Scientists at several leading biotech hubs have demonstrated that mitochondria harvested from human mesenchymal stem cells can successfully repower damaged cells across multiple mammalian species. The implications are staggering: a single stem cell line might one day serve as an off-the-shelf energy source for failing hearts, degenerating neurons, or aged tissues regardless of species barriers. This represents a paradigm shift from personalized mitochondrial therapies to a potentially universal energy solution.
What makes stem cell-derived mitochondria uniquely adaptable? Research points to their immature metabolic profile and reduced immunogenicity compared to adult cell sources. Unlike mitochondria from differentiated tissues, these organelles appear to lack the surface markers that typically trigger immune rejection. Animal trials show particular promise in cardiac applications, where transplanted stem cell mitochondria improved heart function in pigs by 38% post-heart attack – without immunosuppression.
The technology's development hasn't been without controversy. Some ethicists question whether creating interspecies mitochondrial chimeras could have unforeseen consequences. However, proponents argue that since mitochondria have their own DNA separate from the nucleus, the ethical considerations differ substantially from genetic modification of nuclear DNA. Regulatory agencies are currently developing new frameworks to address these unique biological hybrids.
Commercialization efforts are already underway, with three biotech startups racing to scale production of what they term "mitochondrial cartridges" – standardized mitochondrial preparations derived from stem cell banks. The first human trials for refractory epilepsy could begin as early as next year, leveraging mitochondria's role in neuronal energy metabolism. Meanwhile, veterinary applications are progressing faster, with canine trials showing remarkable success in treating age-related mitochondrial decline.
Beyond medicine, the technology raises fascinating evolutionary questions. The original mitochondrial symbiosis between primitive cells and bacteria occurred billions of years ago. Now, science may be enabling a second great mitochondrial adaptation – this time consciously engineered. Some theorists speculate this could lead to enhanced species with optimized energy metabolism, though such applications remain firmly in the realm of speculation for now.
The road ahead presents both extraordinary promise and complex challenges. Manufacturing sufficient quantities of therapeutic-grade mitochondria remains technically demanding, and long-term effects of cross-species mitochondrial transfer require careful study. Yet as research progresses, Mitochondrial Transplantation 2.0 appears poised to transform how we treat energy-deficient diseases across the tree of life – blurring the lines between species at the most fundamental cellular level.

By /Jul 28, 2025
By /Jul 28, 2025
By /Jul 28, 2025
By /Jul 28, 2025

By /Jul 28, 2025

By /Jul 28, 2025
By /Jul 28, 2025
By /Jul 28, 2025

By /Jul 28, 2025
By /Jul 28, 2025

By /Jul 28, 2025
By /Jul 28, 2025
By /Jul 28, 2025

By /Jul 28, 2025

By /Jul 28, 2025
By /Jul 28, 2025

By /Jul 28, 2025
By /Jul 28, 2025

By /Jul 28, 2025
By /Jul 28, 2025