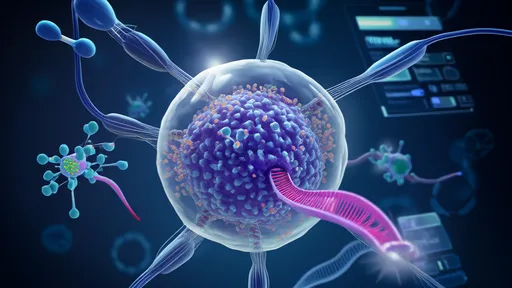
In the relentless battle against antibiotic-resistant superbugs, scientists have turned to nature’s own precision weapon—bacteriophages. These microscopic viruses, which exclusively target and destroy bacteria, are now at the forefront of a revolutionary approach: directed evolution. By harnessing the power of phage therapy and accelerating their adaptation through cutting-edge techniques, researchers are crafting what some call "intelligent missiles" to combat deadly infections where traditional antibiotics fail.
The rise of superbugs like MRSA, Klebsiella pneumoniae, and carbapenem-resistant Pseudomonas aeruginosa has pushed modern medicine to the brink. With antibiotic development stagnating and resistance spreading faster than new drugs can be produced, phage therapy—once a forgotten relic of early 20th-century medicine—is experiencing a renaissance. Unlike broad-spectrum antibiotics, phages are exquisitely specific, evolving alongside their bacterial prey. This inherent adaptability is now being turbocharged in labs worldwide through directed evolution, a process that mimics natural selection but at warp speed.
How does it work? Scientists isolate phages with desirable traits, such as the ability to penetrate bacterial biofilms or evade immune defenses, then subject them to controlled mutations. Using high-throughput screening, they identify the most potent variants and repeat the cycle, effectively "training" phages to become even deadlier to their targets. Recent breakthroughs include phages engineered to target multiple bacterial strains simultaneously or to deliver CRISPR-Cas systems that edit bacterial DNA, inducing lethal mutations.
One landmark study published in Nature Biotechnology demonstrated how directed evolution created phages capable of eliminating E. coli infections in mice within hours—even when the bacteria carried resistance genes. Another team at the Massachusetts Institute of Technology developed "phage-antibiotic synergy" combos, where evolved phages weaken bacterial defenses, allowing conventional antibiotics to regain their potency. These innovations hint at a future where bespoke phage cocktails could be tailored to individual patients’ infections, much like precision oncology.
Yet challenges remain. Regulatory frameworks struggle to categorize phage therapies—are they drugs, biologics, or something entirely new? Manufacturing standardized phage preparations is complex due to their dynamic nature, and some clinicians worry about potential off-target effects. Nevertheless, startups like Adaptive Phage Therapeutics and BiomX are already advancing clinical trials, with promising results against chronic prosthetic joint infections and cystic fibrosis-related superbugs.
As the post-antibiotic era looms, phage-directed evolution offers a glimmer of hope. By merging ancient viral warfare with synthetic biology, scientists aren’t just fighting superbugs—they’re outsmarting them at their own evolutionary game. The "intelligent missile" metaphor may prove apt: these phages don’t carpet-bomb the microbiome but strike with surgical precision, leaving bystander cells unharmed. In hospitals where pan-resistant bacteria claim lives daily, that precision could mean the difference between life and death.

By /Jul 28, 2025
By /Jul 28, 2025
By /Jul 28, 2025
By /Jul 28, 2025

By /Jul 28, 2025

By /Jul 28, 2025
By /Jul 28, 2025
By /Jul 28, 2025

By /Jul 28, 2025
By /Jul 28, 2025

By /Jul 28, 2025
By /Jul 28, 2025
By /Jul 28, 2025

By /Jul 28, 2025

By /Jul 28, 2025
By /Jul 28, 2025

By /Jul 28, 2025
By /Jul 28, 2025

By /Jul 28, 2025
By /Jul 28, 2025